Even though the communications pause has been lifted, people are still asking for recall updates. There was not much recently and I have been really busy so I am sorry for the late post. This is current as of 4/19/25 at 11AM EDT.
Supplement Manufacturing Partner, Inc. Issues Recall on Dorado Nutrition Brand Spermidine Supplement 10mg Vegetable Capsules (Spermidine 3HCL) Due To Undeclared Wheat Allergen
Supplement Manufacturing Partners, Inc. is recalling Dorado Nutrition brand Spermidine Maximum Strength 10 MG per serving, because it contains undeclared wheat. People who have an allergy or severe sensitivity to wheat run the risk of serious or life-threatening allergic reaction if they consume this product.
Spermidine Maximum Strength 10 MG per serving was sold online at Amazon from 05/22/2024 to 04/04/2025.
Product was also sold in Germany as Spermidin 60 Kapseln 38g under Deep Green GmbH aka Space Garden.
The Dorado Nutrition brand Spermidine Maximum Strength 10mg per serving is packaged in a white capsule bottle containing 120 capsules. The product is labeled with a blue outlined label, with a best by date of 04/2026 located on the bottom of the bottle, with Lot Number 12792402-44 and 12792402-44J. No allergic or adverse reactions have been reported to date.
The recall was initiated after a Supplement Manufacturing Partner investigation following a test of the product. It was discovered that product containing wheat was distributed in packaging that did not reveal the presence of wheat. The mislabeled product has been removed from sale.
Consumers who have purchased the product are urged to return them to the place of purchase for a full refund. Consumers with questions may contact the company at SMPQuality@smpnutra.com or at 833-810-9896, 9-5 EST.
May Flower International Inc., Issue Allergy Alert on Undeclared Wheat in “Beijing Soybean Paste”
May Flower International Inc of Maspeth, NY, is recalling its 8.82-ounce/250g packages of “Beijing Soybean Paste” food treats because they may contain undeclared wheat. People who have allergies to wheat run the risk of serious or life-threatening allergic reaction if they consume these products.
The recalled “Beijing Soybean Paste” were distributed nationwide in retail stores.
The Beijing Soybean Paste is packaged in 8.82-ounce/250g plastic packages, with UPC 6917799000385. The labeling indicates that the product was packed For Fu Xiang Yuan Trading Inc New York City NY 11378. (Please see attached photo.) The product is distributed between 03/25/2023 to 03/05/2024 by May Flower International Inc.
No illnesses or allergic reactions involving this product have been reported to date.
This recall was initiated after the United States Food and Drug Administration discovered during a routine inspection that the product declared flour as an ingredient, but wheat (an allergen) was undeclared.
Consumers who have purchased 8.82-ounce/250g packages of “Beijing Soybean Paste” are urged to return them to the place of purchase for a full refund. Consumers with questions may contact the company on 1-718-366-8668 Monday to Friday 9:00AM to 6:00PM Eastern Time (ET).
FDA Classifies Q’Apel Medical Inc.’s Worldwide Medical Device Recall and Discontinuation of its 072 Aspiration System (Hippo) as Class I
Fremont, CA – April 18, 2025 – On April 7, 2025, the U.S. Food and Drug Administration (“FDA” or “the agency”), classified Q’Apel Medical, Inc.’s (“Q’Apel” or “the company”) voluntary recall of 1,617 units of its 072 Aspiration System (also known under the product name “Hippo”, which includes “Cheetah”; collectively, the “product”) as Class I.
On February 26, 2025, the company initiated a discontinuation and recall of 1,617 units of its 072 Aspiration System product. The recall was initiated because the company received a Warning Letter from FDA that raised questions about whether the features and characteristics of the distal tip of the Hippo aspiration catheter were within the scope of its 510(k) clearance. Rather than pursue a new regulatory pathway, the company chose to voluntarily remove all affected product lots and discontinue the 072 Aspiration System line as part of its strategic shift toward newer technologies
Q’Apel has submitted three Medical Device Reportable events for the Hippo product to date; these adverse events include a reported tip detachment, retrieved without patient injury; a vessel rupture; and a vasospasm. Based on the company’s investigation of these events, factors other than the device’s distal tip likely contributed to the reported adverse events.
Notably, each of these event types is a known risk associated with use of any aspiration catheter and is not unique to the Hippo product. The tip of any aspiration catheter used during a thrombectomy could potentially cause vasospasm and/or vascular injury. In particular, if the tip of an aspiration catheter triggers irritation of the vessel wall, it may manifest as vasospasm, which may be self-limiting or may require treatment (e.g., vasodilatation). Vascular injury may result in a non-flow limiting dissection that requires no intervention and causes no permanent morbidity, a flow-limiting dissection that requires intervention and may be associated with morbidity, or in the extreme case, vessel perforation or rupture which requires intervention and likely results in morbidity or even mortality. If unretrieved, a detached tip of a catheter could result in serious adverse events such as blockage of blood vessels, ischemia of end organs, and death.
The product was distributed in the United States, Qatar, United Arab Emirates, and the Republic of Kazakhstan.
The following product configurations have been removed and discontinued:
072 Aspiration System (Hippo with Cheetah Delivery Tool) with Aspiration Tubing; Catalog Number: APT6072-132; Unique Device Identifier: 00857545008127; Lots: FG241008C-03, FG240916C-04, FG240905C-04
072 Aspiration System (Hippo with Cheetah Delivery Tool); Catalog Number: AP6072-132; Unique Device Identifier: 00857545008097; Lots: FG241206A-03, FG240917A-01
072 Aspiration Tubing; Catalog Number: APT-95; Unique Device Identifier: 00857545008103; Lot: FG241206A-04
Actions to be taken by user:
The Hippo product configurations described above have been discontinued in all markets. The company has proactively notified all customers and distributors and will continue to monitor the situation closely. Consignees of the product should immediately return any available product inventory to the company. If consignees have distributed the product to others, contact Q’Apel so that the company can notify them of this action and retrieve any remaining product.
For questions or assistance with product return, please contact:
Q’Apel Medical Inc. – Customer Service Phone: 510-738-6255
Email: orders@qapelmedical.com
Hours: Monday – Friday, 8:00 a.m. to 5:00 p.m. PT
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
Complete and submit the report Online: www.fda.gov/medwatch/report.htm
Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
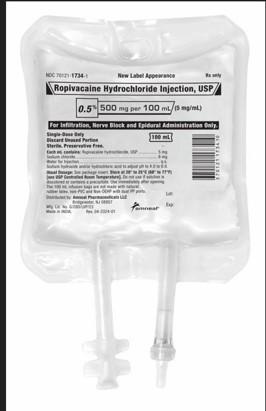
Amneal Pharmaceutical LLC Issues a Nationwide Recall of Ropivacaine Hydrochloride Injection, USP 500mg/100mL, Due to the Potential Presence of Particulate Matter
FOR IMMEDIATE RELEASE – 04/18/2025 – Bridgewater, NJ, Amneal Pharmaceutical LLC, is recalling two lots of Ropivacaine Hydrochloride Injection, USP, 500mg/100mL, Infusion bags to the hospital/user level as the products may contain an inert fiber identified as polypropylene fibers from the IV bag.
Risk Statement: Introduction of polypropylene particulates into the epidural space (or inadvertent administration into the intrathecal space) may result in a variety of adverse events. There is a reasonable probability that particulate matter in the epidural space may cause an epidural inflammatory process to meningitis or potentially damage the spinal cord. Administered intrathecally, particulate matter could result in inflammation, hydrocephalus (water on the brain), which could lead to embolization and organ damage.
To date, Amneal Pharmaceuticals has received no reports of adverse events or injuries related to this recall.
The recalled product was distributed nationwide to wholesalers/distributors between the dates of 04/23/2024 to 11/8/2024 only.
The product is indicated for the production of local or regional anesthesia for surgery and or acute pain management and is packaged in 12x100mL Single Dose IV bags (NDC 70121-17343). The affected Ropivacaine Hydrochloride Injection, USP, 500mg/100mL, products are Lot AL240003 (exp 01/2026) and Lot AL240004 (exp 01/2026). No other Ropivacaine Hydrochloride Injection, USP lots are impacted.
Amneal is notifying its customers by UPS and is arranging for return of all recalled products. Wholesalers/distributors are asked to notify their hospital/ user customers of the recall and provide instruction to contact Amneal for the return of the recalled products to Amneal.
Hospitals/users with questions regarding this recall can contact Amneal Pharmaceuticals by:
Phone: 833-582-0812 Monday-Friday, 8:00 am-5:00 pm, EST
Fax: 631-983-2595
E-mail to: RopivacaineHCl-Recall@amneal.com
For Medical Inquiries or to report Adverse Events, or quality problems experienced with the use of this product, please contact Amneal Drug Safety by phone at 1-877-835-5472, Monday - Friday, 8:00 am – 6:00 pm, EST, or e-mail at DrugSafety@amneal.com.
Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
Complete and submit the report Online: www.fda.gov/medwatch/report.htm
Regular Mail or Fax: Download form www.fda.gov/MedWatch/getforms.htm or call 1800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178
This recall is being conducted with the knowledge of the U.S. Food and Drug Administration
Recall Reminder: Gerber Products Company Previously Recalled and Discontinued All Batches of Gerber® Soothe N Chew® Teething Sticks Due To Potential Choking Hazard
ARLINGTON, Va., April 18, 2025
On January 31, 2025, Gerber Products Company initiated a recall and discontinuation of all batches of GERBER® SOOTHE N CHEW® TEETHING STICKS due to a potential choking hazard for babies and young children.
We are issuing a second press release about this recall due to recent reports of recalled product still available for sale on some retailer shelves and online. The previous announcement on January 31st is linked hereExternal Link Disclaimer and on FDA’s recall page.
GERBER® SOOTHE N CHEW® TEETHING STICKS were distributed nationwide.
Recalled products can be identified as follows:
Gerber Snacks for Baby soothe ‘n’ chew Teething Sticks, Strawberry Apple, Net Wt 3.2 Oz (90g), with UPC 0 15000 04618 7, all lot codes
Gerber Snacks for Baby soothe ‘n’ chew Teething Sticks, Banana, Net Wt 3.2 Oz (90g), with UPC 0 15000 04608 8, all lot codes
Gerber Snacks for Baby soothe ‘n’ chew Teething Sticks, Banana, Net Wt 1.58 Oz (45g), with UPC 0 15000 01015 7, all lot codes
Gerber® Soothe N Chew® Teething Sticks - Product Packaging -See Images Below
The previously issued recall and discontinuation is isolated to GERBER® SOOTHE N CHEW® TEETHING STICKS – STRAWBERRY APPLE and GERBER® SOOTHE N CHEW® TEETHING STICKS – BANANA.
The recall was initiated after receiving consumer complaints of choking incidents.
Consumers who may have purchased GERBER® SOOTHE N CHEW® TEETHING STICKS should not feed this product to their child and can return the product to the retailer where it was purchased for a full refund. Consumers who find the product for sale in the market should not purchase the product. Anyone with health concerns should contact a health care provider. For any additional support needed, Gerber is available 24/7 at 1-800-4-GERBER (1-800-443-7237).
We have been working with the U.S. Food & Drug Administration (FDA) on this recall and will cooperate with them fully.
Again, we sincerely apologize for any concern or inconvenience this action represents to parents, caregivers and retail customers.
Link to Initial Recall
Harvest NYC Inc Recalls Enoki Mushroom Due to Possible Health Risk
Harvest NYC Inc of Brooklyn, NY 11231 is recalling its 200g packages of Enoki Mushroom, because they may be contaminated with Listeria monocytogenes, an organism which can cause serious and sometimes fatal infections in young children, frail or elderly people and others with weakened immune systems. Although healthy persons may suffer only short-term symptoms such as high fever, severe headache, stiffness, nausea, abdominal pain, and diarrhea, Listeria infection can cause miscarriages and stillbirths among pregnant women.
The recalled Enoki Mushrooms were distributed nationwide in retail stores. The product comes in a 200g, green plastic package marked with UPC Barcode 6975730520101 on the back label, distributed by Hofood99 Inc., 21903 56th Ave Oakland Gardens, NY 11364.
No illnesses have been reported to date in connection with this problem.
The contamination was discovered after samples were collected from a store in Buffalo, NY and subsequent analysis by NYS Department of Agriculture and Markets Food Laboratory revealed the presence of Listeria monocytogenes in some 200g packages of Enoki Mushroom.
Consumers who have purchased 200g packages of Enoki Mushroom from January 11- 31, 2025 are urged to destroy the products immediately or return them to the place of purchase for a full refund. Consumers with questions may contact the company at (718) 596-0777.
Subscribe to stay informed on your health. Although not required, any support is greatly appreciated.














Thank you thank you thank you times a million for posting these & helping everyone stay safe! 💖💖💖
Thank you once again for keeping all of us so well informed.